The medical field is driven by innovation. Groundbreaking devices and components are conceived every day. Unfortunately, many companies, engineers, and entrepreneurs don’t know how to turn their life-saving ideas into a functional product. Additionally, the regulatory environment and industry quality requirements are complex and difficult to navigate.
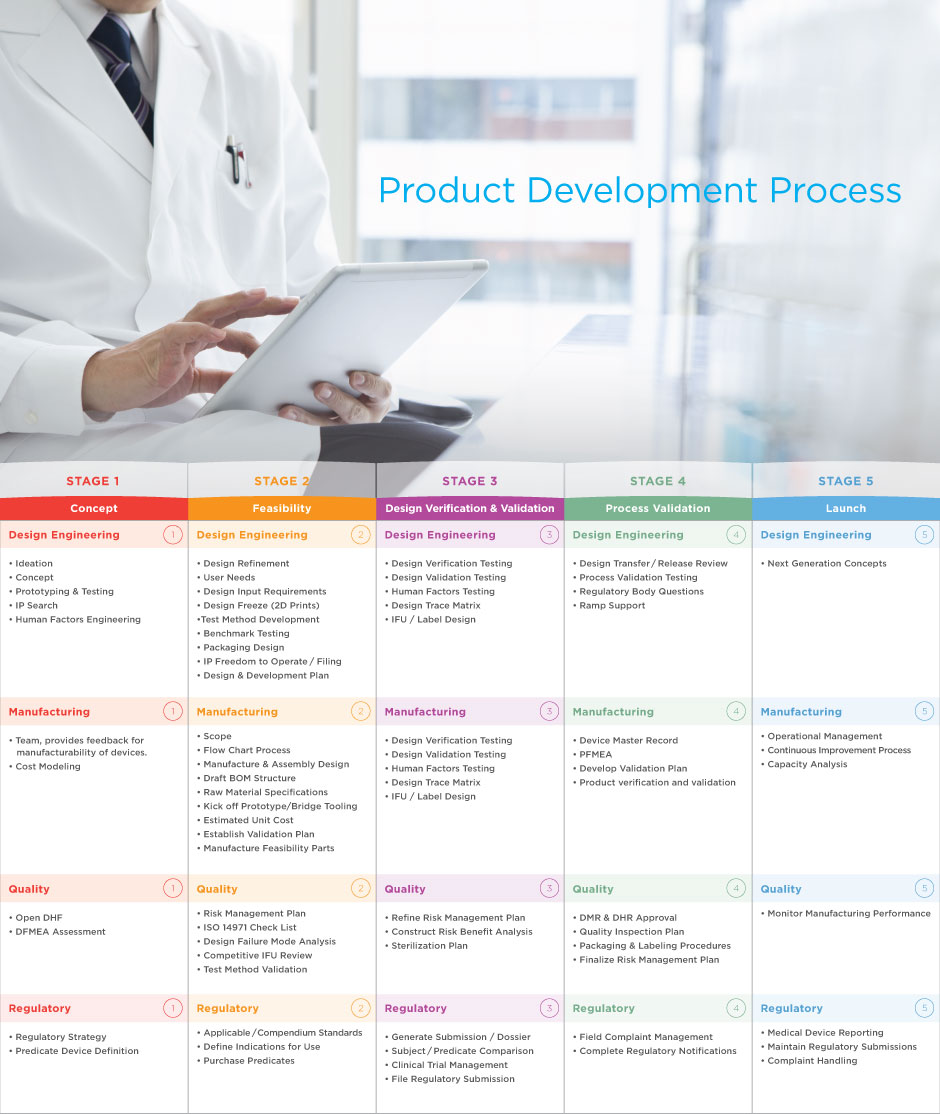
The Biomerics Product Development Process provides a systematic, stagegate approach to new product development and commercialization. The chart above outlines the key design engineering, quality, manufacturing, and regulatory deliverables for each stage in the process. We look forward to working with you to develop a specific plan for your unique product.





