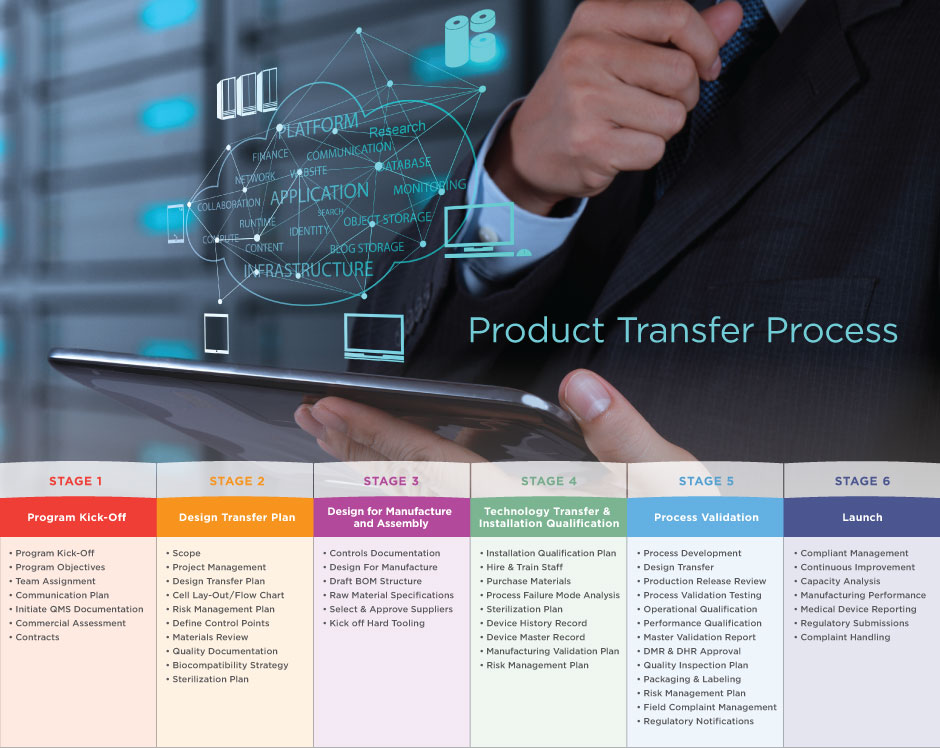
Many of the world’s leading medical device companies contract with Biomerics to manufacture their devices. We have established an ISO 13485 and FDA 21 CFR Part 820 compliant Product Transfer Process to ensure that all manufacturing, quality, and regulatory requirements are met. Our process allows us to manage anything from a single product line to a full facility transfer.
The Biomerics Product Transfer Process enables us to partner with customers to ensure their process validation requirements are met. During this process, we assign a dedicated, cross-functional team that includes design engineering, quality engineering, process engineering, and regulatory support. This team works directly with the customer to establish all documentation and confirm that program requirements are satisfied. The chart above outlines the key deliverables for each stage in the Product Transfer Process.